Quality Assurance For Sterile Products
Ity Assurance and Six Sigma. Items are recycled for sterile reprocessing and feedback is summarized.

Pdf Pharmaceutical Microbiological Quality Assurance And Control Practical Guide For Non Sterile Manufacturing
Simple changes can help.
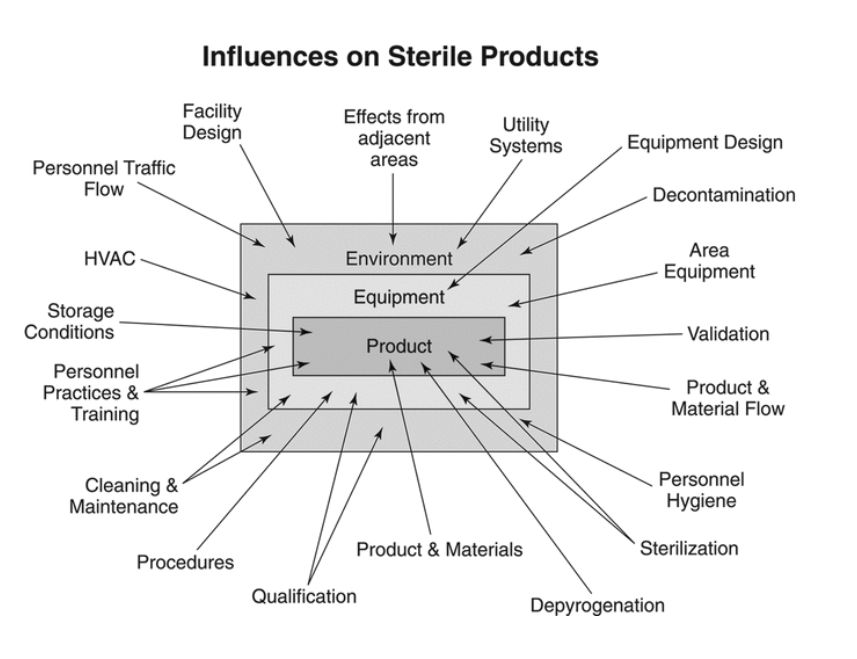
Quality assurance for sterile products. Sterility assurance monitoring is a vital component of your facilitys overall quality assurance program. Akers Informa Healthcare 2010. Entirely free from living organisms of all types.
Comment in Am J Health Syst Pharm. Pharmacists are urged to. The test should be validated for the products concerned.
1Center on Pharmacy Practice Management ASHP Bethesda MD 20814 USA. 2006 on behalf of NHS Pharmaceutical Quality Assurance Committee. The following is a list of sterile product profile classes effective at the time this program was.
Quality assurance for sterile products. However can the assurance of sterility of a medicinal product only be demonstrated by passing the sterility test. The results of a survey of hospital pharmacy and home infusion facility activities for quality assurance in the preparation of sterile drug products are reported and the hospital results are compared with those of a 1991 ASHP survey.
An alternate definition is the operational techniques and. Quality control 21 The sterility test applied to the fi nished product should only be regarded as the last in a series of control measures by which sterility is assured. A method to match quality assurance and quality con trol activities with the potential risks to patients posed by various types of products.
CHAPTER 56 DRUG QUALITY ASSURANCE SUBJECT. JulAug 2001 - Sterile Product Compounding View All Articles in Issue. 79 products and sterile active substances via adaption using the principles of quality risk 80 management QRM to ensure that microbial particulate and pyrogen contamination 81 associated with microbes is prevented in the final product.
Formulation Manufacture and Quality Assurance 1 - 2 March 2016 Course Topics Include. Quality Audits for Processed Sterile Devices Lesson No. 1206 Sterile Drug.
Quality Assurance of Aseptic Preparation Services. Sterility assurance products including biological indicators BI and chemical indicators CI provide you the confidence that the sterilizer is functioning properly and cycle conditions are adequate to produce medical devices that are ready and safe for patient use after reprocessing. History Since the inauguration of sterility test.
The sterility test is mandatory for all sterile medicinal products and remains an important criterion for product release by demonstrating that the finished product is sterile ie. ASHP guidelines on quality assurance for pharmacy-prepared sterile products. Santell JP1 Kamalich RF.
The rules governing medicinal products in the European Community. Formulation and M anufacture of Solutions Suspensions and Lyophilized Products. Good Manufacturing Practice for medicinal products.
Am J Hosp Pharm. Reactive Audits those done by user de-partment personnel. Only those techniques with importance either to microbiology or the confirmation of sterility of the final product are.
American Society of Health System Pharmacists. Skills and Environmental Quality and Control1 End-preparation sterility test-ing falls under the Finished Preparation Release Checks and Tests section1 Each section is important but the focus here is only on sterility testing. General areas of Quality control Three General Areas are.
643 rows Quality Assurance for Sterile Products. Routine work testing 2. The focus on developing implementing and using quality systems during pharmacy preparation of sterile products has never been important than it is todayUnited States Pharmacopeia USP Chapter.
A questionnaire was mailed in May and June 1995 to 778 hospital p. IMPLEMENTATION DATE September 11 2015. Division of Microbiology Assessment I.
Information on quality assurance and quality control activities that should be applied to the preparation of sterile products in pharmacies and 2. Quality control can be defined as part of quality management focused on fulfilling quality requirements While quality assurance relates to how a process is performed or how a product is made quality control is more the inspection aspect of quality management. It is not the aim of this section to review the entirety of quality control of sterile products or of the chemical assays and requirements of drugs and excipients prior to formulation.
Course Director Course offered by STERILE PRODUCTS. QUALITY CONTROL AND QUALITY ASSURANCE. Include numerable tests reading and observations through out the manufacturing process 3.
National survey of quality assurance activities for pharmacy-prepared sterile products in hospitals and home infusion facilities--1995. Kastango Eric S Douglass Kate. Central Service Technical Manual.
79 December 2004. 22 Samples taken for ster ility test ing should be representat ive of the whole. 12 Sterilization and sterility assurance 147 121 Introduction 147 122 Sterility 147 123 Sterility assurance and the sterility assurance level 148 124 Sterility testing 150 125 Parametric release 151 126 Sterile products 152 127 Sterilization 154.
Formulation Packaging Manu-facturing and Quality by Michael J. Kastango Eric S Douglass Kate Issue. Lau D Shane R Yen J.
Sterility test Pyrogen test Clarity test Leaker test 24. Quality Assurance for Sterile Products.

Pin By Boobuli Bushki On Books To Read In 2021 Pharmaceutical Cool Designs Design

Infographics Design Template Infographic Design Design Template Infographic

Layout For Injection Manufacturing Unit Pharmaceutical Guidelines Pharmaceutical Manufacturing Layout Warehouse Design Layout

Microbial Quality Assurance In Pharmaceuticals Cosmetics And Toiletries 1st Edition Ebook Rental In 2021 Pharmaceutical Baird Cosmetics And Toiletries

Quality Control And Assurance Ppt Download

Surgical Instrument Processing Certificate Program Nursing School Scholarships Importance Of Time Management Surgical Technician

Pin On Quality Assurance Product

Safe Drugs Happy Consumers Sterility Assurance Programs The American Association Of Homeopathic Pharmacists

Lantian Ce Certifaction Medical Supplies Medical Surgical Gown

Quality Assurance Fundamentals In Good Manufacturing Practices Gmpsop

Pdf Quality Assurance For Sterile Products

Practice Basics Chapter 16 Aseptic Technique Sterile Compounding

Pdf Quality Assurance For Sterile Products

Sterikit Manufacturing Facility Color Change Manufacturing

Qualification Of Hvac Systems Hvac System Qualifications System

Manufacturing Implementation And The Pharmaceutical Quality System Pharmacy Technician Study Case Study Pharmacy Technician



Posting Komentar untuk "Quality Assurance For Sterile Products"