Human Subject Assurance Training
Clinical investigations that involve the use of drugs biologics or deviceswhether unlicensed or used outside standard medical practiceare subject to IRB review and approval under 21 CFR Part 50 external icon and 21 CFR Part 56 external icon. Resources including the course content from the retired PHRP course the Human Subjects Research and Exempt Human Subjects Research infographics funding opportunity announcements bioethics information links to OHRP and.

Fwa Irb Registrations Ucla Office Of The Human Research Protection Program
For United States OHRP.

Human subject assurance training. The Collaborative Institutional Training Initiative CITI Program proved themselves committed to promoting the publics trust in the research enterprise by providing high quality peer-reviewed web-based educational courses in research ethics regulatory oversight responsible conduct of research research administration and other topics pertinent to the interests of member. A printable completion certificate is available at the conclusion of the lesson so. HHSs Interactive Training Video.
GCP training is not the same as human subjects protections and is not covered in the basic courses in human subjects protections offered in CITI. This can be achieved by completing OHRPs Human Research Protection TrainingPlease review NIHs Educational Requirement for additional information. For NIH-funded human subjects research investigators and all key personnel must fulfill the protection of human subjects education requirement.
View the educational webinars on the Department of Health and Human Services HHS regulations requirements for the protection of human subjects. Access these role-based resources on our HRPP Resources page. Human Subject Assurance Training.
Just Now Hhsgov Related Courses. United States Office for Human Research Protections. Human Subjects Ethics Training All investigators faculty staff and students are required to complete the CITI Program training in human subjects protection prior to conducting research using human subjects.
Human Research Protection Training HHSgov. This lesson introduces human research protections the Common Rule and the offices and agencies that oversee of human subjects research. Film or Broadcast.
IHS recommends that this training also be taken by Chief Executive Officers or Service Unit Directors of all IHS facilities where human research is being conducted. Read the Human Subjects Research Training SOP. Then go to Step 2 below to find the link to the education.
NIH Required Human Subjects Training. The Research Clinic is an interactive training video educates clinical and social researchers on the importance of appropriately protecting research subjects. Please read the Belmont Report as it forms the basis of the Marshall University Human Research Protection Program.
All National Institutes of Health NIH grant and contract awards that involve human subjects issued after October 1 2000 require signed institutional documentation that all key personnel on a given award have received educationtraining in the protection of human subjects. Office for Human Research Protections OHRP Webinars. Human Research Protection TrainingOHRP offers this comprehensive training for the research community on human research protections based on the requirements of the revised Common Rule or the 2018 Requirements.
In order to qualify for an Assurance with OHRP the OHRP strongly recommends that Institutional Officials complete Module 1 of the Human Subjects Assurance Training web-based from OHRP. This written commitment is called an Assurance of Compliance. Human Subjects Protection Training.
Just Now Hhsgov Related Courses. Human Research Protection Training HHSgov. Submit completed Planned Enrollment Report and Cumulative Inclusion Enrollment Report when appropriate.
Women Minorities and All Ages. Once you have read the report print the following instructions that are applicable to the IRB to which you will submit. Required for all individuals involved in human subject research studies exempt and non-exempt who have contact with subjects or their identifiable data.
Training including required training information for completing applications training for using the Human Subjects System HSS and Single IRB training. HRPO facilitates the work of the IRB and provides assistance and training for CDC staff engaged in research involving human participants. For additional training OHRP also offers compiled lists of helpful resources specific to professional roles.
The Collaborative Institutional Training Initiative CITI online training is used to fulfill this requirement. If you are involved in the conduct oversight or management of an NIH funded clinical trial you will need to complete this training in addition to any other training that is required by UI policy or your department. For human subjects research conducted or supported by the Department of Health and Human Services HHS the Office for Human Research Protections OHRP must approve an institutions Assurance before the human subjects research can begin.
5 hours ago OHRP offers online educational materials to help IRB members and administrators investigators institutional officials and others better understand the HHS regulations for the protection of human subjects in research and their. Human Hhsgov Related Courses. Provide certification of human subjects education for key personnel or send just-in-time at the time of award.
Human subjects protections phs act federalwide assurance FWA HHS office for human research protections OHRP guidance on engagement of institutions in human subjects research IRB certification of IRB approval reporting to funding agency and OHRP OHRP oversight education in the protection of human research participants data and safety monitoring inclusion of children as. 5 hours ago Online Education HHSgov. Learn more about Inclusion of Special Populations.

Quality Assurance Is A Planned And Systematic Means For Assuring Management That The Defined Stand Project Management Tools Change Management Quality Assurance

Training Requirements Research Integrity And Assurance

Life Assurance Physical Differences Between Men And Women Regarding Training Pelvis Anatomy Pelvic Girdle Skeleton Anatomy

Training Requirements Research Integrity And Assurance

The Human Research Protection Program Hrpp Office Of The Senior Vice President For Research At Penn State
Human Research Protection Program Hrpp Ucsf Institutional Review Board

Human Subjects Research Office Of The Vice Chancellor For Research University Of Illinois Chicago

Training Requirements Research Integrity And Assurance

Training Requirements Research Integrity And Assurance

Human Research Protection Program Hrpp Ucsf Institutional Review Board

Human Subjects Irb University Research Services Administration

Training Requirements Research Integrity And Assurance

Human Subjects Protection Irb Rio Uncw
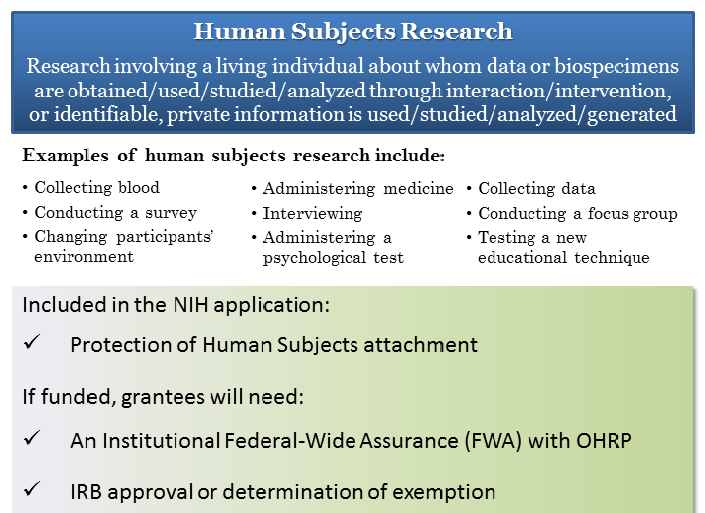
Definition Of Human Subjects Research Grants Nih Gov

The Human Research Protection Program Hrpp Office Of The Senior Vice President For Research At Penn State

Quality Assurance Checklist Personal Development Skills Quality Assurance Process Improvement

Human Research Protection Program Research Integrity And Assurance

Training Research Integrity And Assurance

Posting Komentar untuk "Human Subject Assurance Training"