Msc In Pharmaceutical Quality Assurance And Regulation
A country should address all issues at some level as part of a basic pharmaceutical quality assurance infrastructure. Pharmaceutical Quality Assurance.
Pharmaceutical Data Integrity Issues Challenges And Proposed Solutions For Manufacturers And Inspectors Gabi Journal
Plan select and evaluate the use of modern spectroscopic instrumental chemical and bio -analytical techniques in a pharmaceut ical context 24.

Msc in pharmaceutical quality assurance and regulation. Performed and the data are generated documented and recorded in compliance with Good Clinical Practice and applicable regulatory requirements. School of Chemical and Pharmaceutical Sciences. Pharmaceutical Technology and Quality Assurance MSc Postgraduate Certificate - PgCert Postgraduate Diploma.
Quality assurance is a wide ranging concept covering all matters that individually or collectively influence the quality of a product. Quality assurance and regulatory affairs. Apply now for Kingston University Londons Pharmaceutical Science MSc degree.
Figure 19-1 Quality assurance framework Decision making and enforcement Document review. The Level 8 Certificate in Medical Technologies Regulatory Affairs and Quality provides students with an introduction to the core elements of the product. MSc Pharmaceutical Science Technology Programme Master of Science Pharmaceutical Science Technology NUS Department of Pharmacy has been running the Master of Science Pharmaceutical Science and Technology MPST programme since 2008.
QA is the heart and soul of quality control QA QC. The quality standards appropriate to their intended use and required by drug regulatory authorities. Critical ly assess and evaluate potential drug toxicity 23.
DT233 MSc Pharmaceutical Quality Assurance and Biotechnology Full Time DT237 MSc in Pharmaceutical Quality Assurance and Regulation Part-time DT9279 MSc Pharmaceutical Validation Technology Part-time You are here. It will build a critical and technical knowledge related to development analysis and production of medicines and the drug industry. What is Role of Quality Assurance department in Pharmaceutical Industry.
DT237 MSc in Pharmaceutical Quality Assurance and Regulation Part Time This programme is offered on a two year part-time basis. The planned and systematic actions that are established to ensure that the trial is. Apply appropriate regulatory requirements for.
Personnel from the regulatory authorities and the pharmaceutical industry. Northeasterns MS in Regulatory Affairs for Drugs Biologics and Medical Devices offers students the opportunity to meet their career goals and gain deeper experience in key areas of regulatory affairssuch as operational and strategic regulatory affairs clinical regulatory affairs new gene therapies cybersecurity of medical devices quality assurance in biopharmaceutical product. Changes in Pharmaceutical Regulation 22.
Admissions are open for interested students in MSc Pharmaceutical Quality Assurance and Biotechnology or Pharmaceutical Quality Assurance and Regulation at. Postgraduate Courses for Quality Assurance in the United Kingdom. England Royal Holloway University of London Electronic.
University of Reading School of Chemistry Food and Pharmacy. MSc Pharmaceutical Technology and Quality Assurance Improve your skills and knowledge if you are a pharmacy health or biomedical professional working in the technical services. Food Technology Quality Assurance MSc.
The Pharmaceutical Quality Regulation PG Diploma combines specialist good manufacturing practices GMP and analysis knowledge with an understanding of local and global legislation in order to produce quality professionals who can provide long-term solutions to critical manufacturing defects and are able to work in production planning and inventory control method development and validation. This modular course awarding a Level 9 certificate aims to introduce learners to the quality systems and the regulatory framework that assures the quality safety and efficacy of medicinal products in the pharmaceutical industry. Yimer MEKONNEN Lecturer of pharmaceutical analysis and pharmaceutical quality assurance and regulation expert Cited by 37 of Jimma University Jimma Read.
The standards include criteria for personnel facilities packaging quality control and in most cases stability testing. MSc in Pharmaceutical Quality Assurance and Regulation This programme is offered on a two year part-time basis. An accredited QQI Level 9 postgraduate award to help you grow in the pharmaceutical industry.
It is the totality of the arrangements made with the object of ensuring that pharmaceutical products are of the quality required for their intended use. Learn About the Master of Science Degree in Regulatory Affairs and Quality Assurance RAQA The first university to develop a graduate program in Quality Assurance and Regulatory Affairs Temple School of Pharmacy TUSP continues to set the gold standard in this dynamic professional discipline. MScPgDipPgCert Pharmaceutical Quality Good Manufacturing Practice.
This programme is ideal for those involved in manufacturing compliance business improvement quality assurance. The cohesion of the student group rapidly develops as all participants are generally working in some aspect of quality assurance in the pharmaceutical industry. This course is highly relevant to immediate employment.
Our MSc in Pharmaceutical Technology and Quality Assurance is aimed at pharmacy health and biomedical professionals working in the technical services for the NHS or private sector organisations who want to update their skills and knowledge while gaining an academic qualification. ICH Good Clinical Practice Definition of Quality Assurance. It is designed to consolidate supplement and enhance the knowledge and experience of graduates currently employed in the pharmaceutical sector.
It is designed to consolidate supplement and enhance the knowledge and experience of graduates currently employed in the pharmaceutical sector. Of course a countrys quality assurance system is only as effective as its ability to monitor and enforce regulations. MSc Pharmaceutical Business and Technology is a part.
Continuous Manufacturing Versus Batch Manufacturing Benefits Opportunities And Challenges For Manufacturers And Regulators Gabi Journal

Pdf Regulating Pharmaceuticals In Europe Striving For Efficiency Equity And Quality

Overview Of Regulatory Intelligence Methodology Download Scientific Diagram

Regulatory Operations In The Life Sciences Industry Mastercontrol

Pharmaceutical Science With Regulatory Affairs Bsc Hons Degree Course London Undergraduate Courses Kingston University London

Postgraduate Diploma Pharmaceutical Quality Regulation Ucl School Of Pharmacy Ucl University College London

Exploring The Formal And Informal Roles Of Regulatory Intermediaries In Transnational Multistakeholder Regulation Bres 2019 Regulation Amp Governance Wiley Online Library
Continuous Manufacturing Versus Batch Manufacturing Benefits Opportunities And Challenges For Manufacturers And Regulators Gabi Journal

Pdf Impact Of Regulatory Requirements On Medicine Registration In African Countries Perceptions And Experiences Of Pharmaceutical Companies In South Africa

Pin On Gira Global Institute Of Regulatory Affairs

Pdf How Data Protection Regulation Affects Startup Innovation
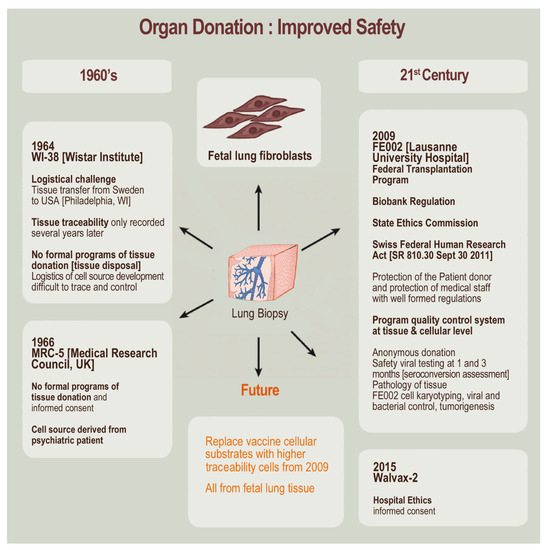
Cells Free Full Text Evolution Of Diploid Progenitor Lung Cell Applications From Optimized Biotechnological Substrates To Potential Active Pharmaceutical Ingredients In Respiratory Tract Regenerative Medicine Html
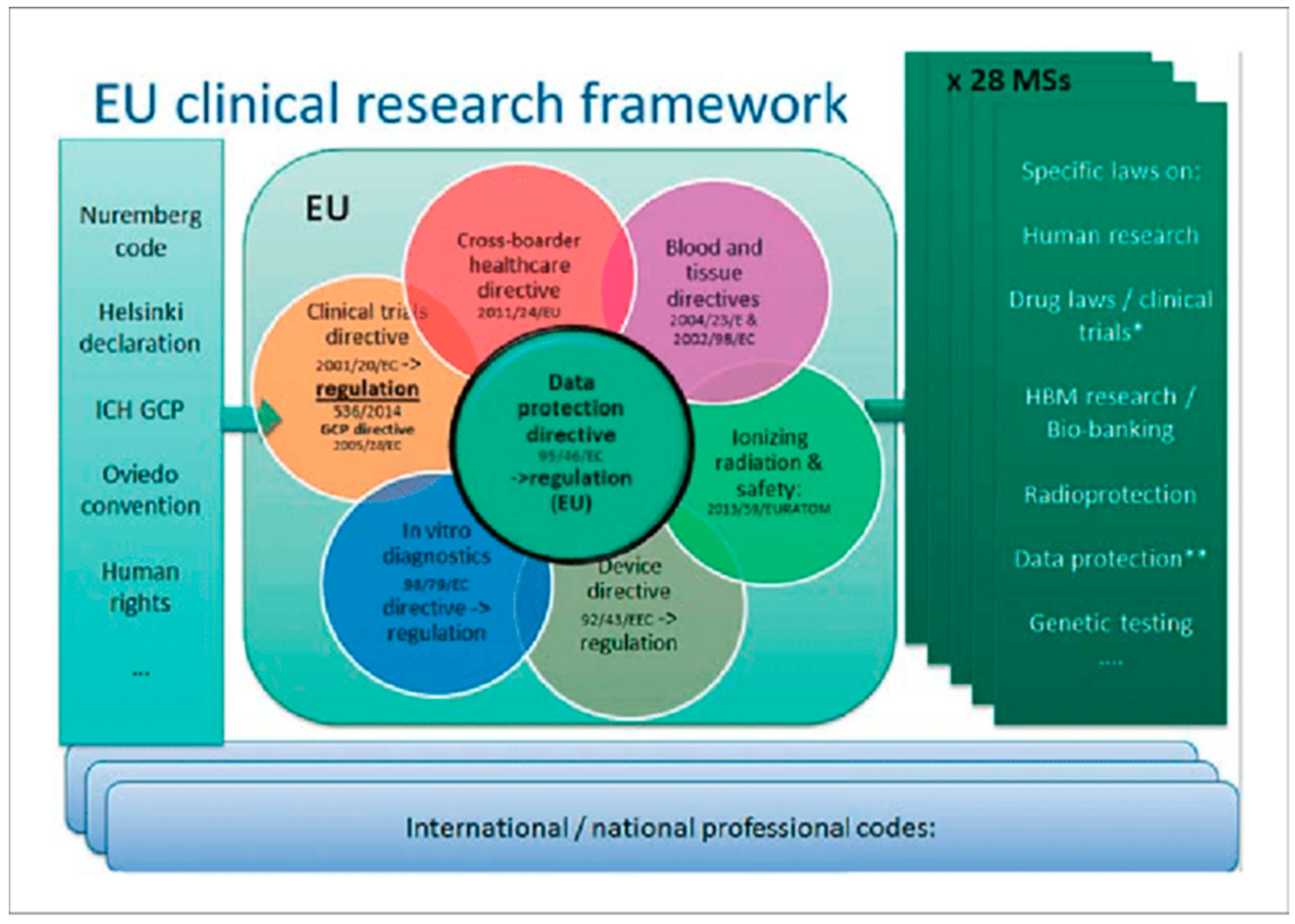
Proceedings Free Full Text Translational Research In Osteogenesis Imperfecta And Cell Therapy Html

Pdf How Data Protection Regulation Affects Startup Innovation
Regulatory Affairs And Quality Assurance Graduate School Opportunities Ivt Regulatory Industry Guidance

Gmp Meets Regulatory Affairs Live Online Training Eca Academy

Regulatory Affairs Vs Quality Assurance Two Career Paths
Posting Komentar untuk "Msc In Pharmaceutical Quality Assurance And Regulation"